Latest news
Recently, the research paper titled "Engineering Crystalline/Amorphous Interfaces for Enhanced CO2 Electroreduction", led by Professor Wang Qiang's team from the College of Environmental Science and Technology has been published in Angewandte Chemie International Edition (IF=16.1), a top-tier chemistry journal.
The extensive use of fossil fuels, such as coal and petroleum, has contributed to societal progress but also resulted in excessive CO2 emissions, worsening global warming and causing severe environmental challenges. CO2 electroreduction is a promising method for reducing these emissions by converting CO2 into highly value-added fuels and chemicals under mild and controllable conditions using renewable electricity. However, CO2 electroreduction reaction (CO2RR) involves complex multi-electron-proton transfer processes, producing a variety of products such as carbon monoxide, formic acid, ethylene, and ethanol, depending on the catalyst employed. Among these products, formic acid is particularly attractive due to its extensive industrial applications in leather, pesticides, and rubber manufacturing, along with its strong market potential. Notably, the production of formic acid through CO2RR achieves the highest economic return per kilowatt-hour of energy input (0.43 $ kWh−1), underscoring its commercial feasibility. Catalysts based on In, Sn, and Bi primarily promote the production of formic acid. However, despite advancements in catalyst design, such as morphology design, defect engineering, and element doping, attaining high selectivity and stability at industrially relevant current densities (≥500 mA cm−2) continues to be a significant challenge.
To tackle these challenges, we propose an amorphization strategy to regulate catalyst reconstruction under operational conditions. In particular, an amorphous In-based catalyst, InOx(OH)3−2x, was designed and achieved high Faradaic efficiencies of 98% at −800 to −1000 mA cm−2 for CO2 electroreduction to formate, while maintaining stability for 100 h. Mechanistic studies show that InOx(OH)3−2x undergoes partial reduction to stable crystalline/amorphous In/In─OH interfaces instead of fully reducing to metallic In, enhancing the adsorption of CO2 and OCHO intermediate. To improve the economic viability of electrolysis system, a CO2 electroreduction coupled with waste plastics electrooxidation system for formate production was constructed. Compared to conventional electrolysis system, the coupled system reduced energy consumption by 34.7% and increased formate production by 49.7%, offering a more energy-efficient and cost-effective approach to CO2 electroreduction.
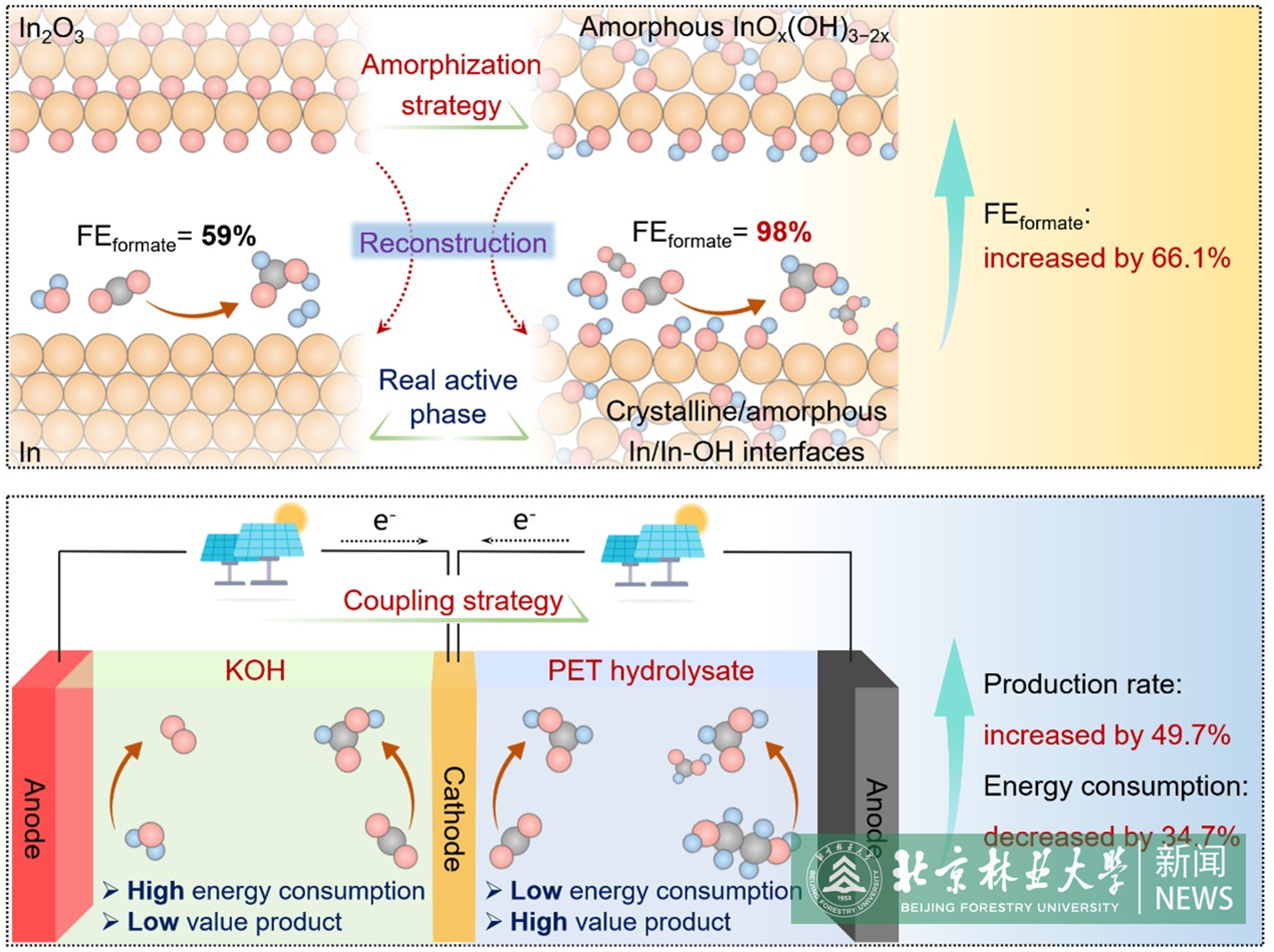
Notably, it has excellent long-term stability, maintaining FEformate above 90% even after 100 h of continuous operation. The characterization results indicate that during CO2 electroreduction, In─O species are preferentially reduced compared to In─OH, leading to the formation of stable crystalline/amorphous In/In─OH interfaces. DFT calculations further elucidate that the presence of the amorphous phase in In/In─OH lowers the CO2 adsorption energy barrier, thus enhancing CO2 adsorption. The interface structure can further optimize charge transfer and facilitate the adsorption of the *OCHO intermediate. We further evaluated the economic viability of coupling CO2 electroreduction with waste plastics electrooxidation to produce formic acid. Experimental results demonstrate that, compared to conventional electrolysis systems, the coupled system reduced energy consumption by 34.7% while increasing formate production by 49.7%. The techno-economic analysis further confirms that this coupling strategy can significantly improve the economic viability of CO2 electroreduction systems, highlighting its potential for large-scale implementation. Moreover, upgrading the products and optimizing the reactor design would further enhance production efficiency.
Li Bingkun, a doctoral student from the College of Environmental Science and Technology, is the first author. Professor Wang Qiang and Associate Professor Xie Wenfu are the corresponding authors. Beijing Forestry University is the first completing institution of the article.
This work was supported by the National Natural Science Foundations of China (No. 52470113 and 52225003), the 5·5 Engineering Research & Innovation Team Project of Beijing Forestry University (No. BLRC2023B04), and the Open Project Fund from Guangdong Provincial Key Laboratory of Materials and Technology for Energy Conversion, Guangdong TechnionIsrael Institute of Technology (No. MATEC2023KF007).
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202509502
Written by Li Bingkun, Xie Wenfu
Translated and edited by Song He
Reviewed by Yu Yangyang












