Latest news
The research paper "Sulfur Changes the Electrochemical CO2 Reduction Pathway over Cu Electrocatalysts" completed by Professor Wang Qiang's research group of the College of Environmental Science and Engineering was published in top journal Angewandte Chemie International Edition (IF=16.6).
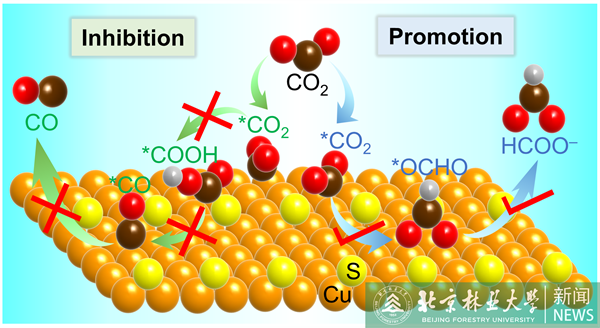
Electrochemical CO2 reduction to value-added chemicals or fuels offers a promising approach to reduce carbon emissions and alleviate energy shortage. Cu-based electrocatalysts have been widely reported as capable of reducing CO2 to produce a variety of multicarbon products (e.g., ethylene and ethanol). In this work, we develop sulfur-doped Cu2O electrocatalysts, which instead can electrochemically reduce CO2 to almost exclusively formate. We show that a dynamic equilibrium of S exists at the Cu2O-electrolyte interface, and S-doped Cu2O undergoes in situ surface reconstruction to generate active S-adsorbed metallic Cu sites during the CO2 reduction reaction (CO2RR). Density functional theory (DFT) calculations together with in situ infrared absorption spectroscopy measurements show that the S-adsorbed metallic Cu surface can not only promote the formation of the *OCHO intermediate but also greatly suppress *H and *COOH adsorption, thus facilitating CO2-to-formate conversion during the electrochemical CO2RR.
Professor Wang Qiang and Lecturer Tianyu Zhang are the co-corresponding authors of the paper, and the first author is Shuyu Liang, a doctoral student of the College. This work was supported by the the National Natural Science Foundation of China(52225003,22208021) and the City University of HongKong start-up fund.
Paper link: https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202310740












