Latest news
Recently, a research paper titled "Unveiling the impurity phase self-promoting mechanism in minerals-derived Li4SiO4 for enhanced CO2 capture performance" by Professor Wang Qiang's research team from the College of Environmental Science and Technology has been published in Chemical Engineering Journal (IF=13.2), a top-tier Q1 journal in materials science.
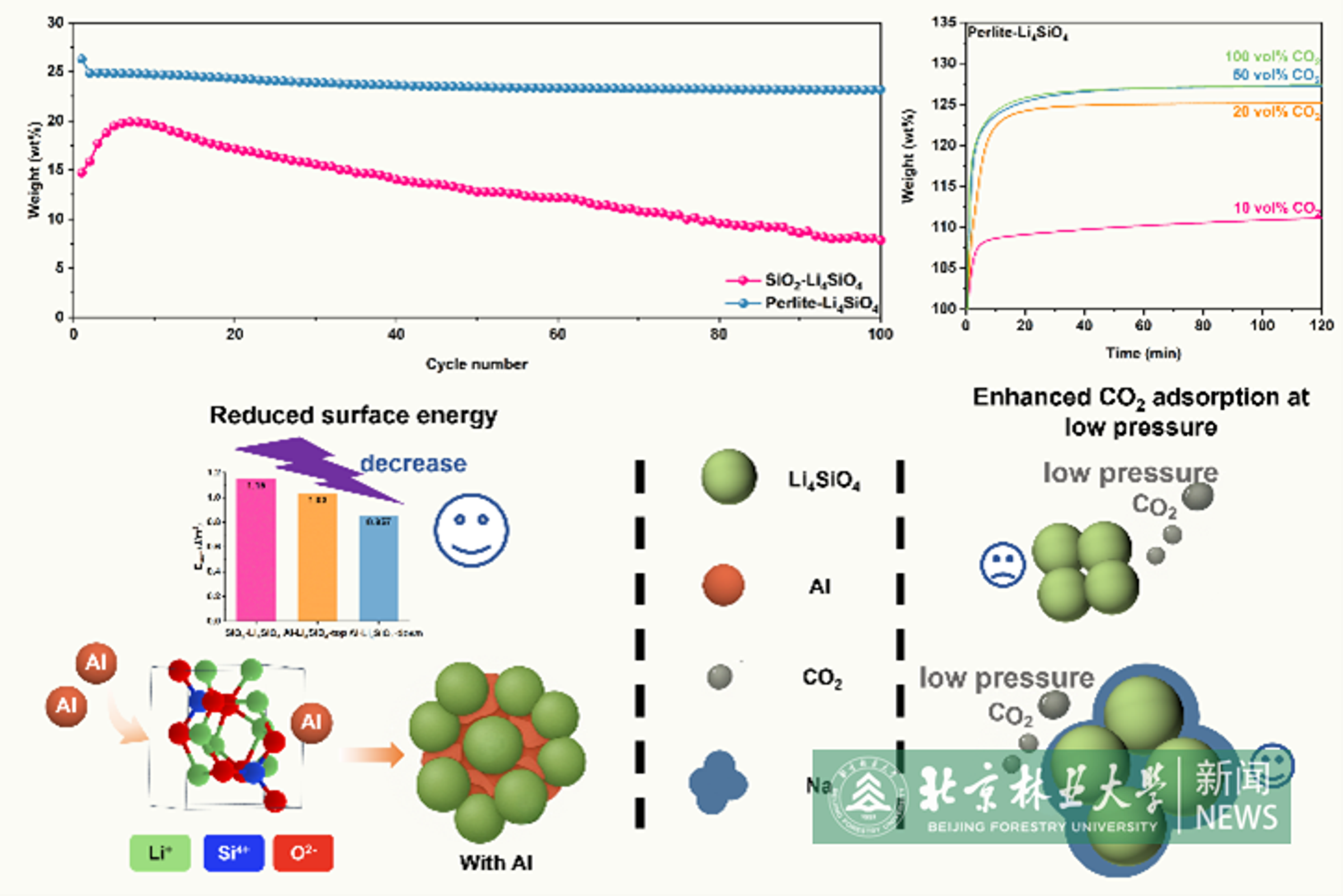
It is widely accepted that carbon dioxide (CO2) is one of the major greenhouse gases for global warming. Since 1880, the global temperature has increased by at least 1.1 °C. The rise in atmospheric CO2 level is primarily attributed to the combustion of fossil fuels in industries. Therefore, capturing CO2 from flue gases is a crucial strategy for mitigating greenhouse gas emissions. Particularly, CO2 capture using solid capturing materials is a very promising technology, distinguished by a wide sorption temperature range, minimal waste generation during regeneration, and environmentally friendly disposal of spent sorbents. The synergistic advantages of enhanced CO2 capture efficiency and operational durability position these materials as promising candidates for industrial-scale decarbonization, generating significant research interest across fundamental studies and commercial deployment. Although minerals-derived Li4SiO4 demonstrates enhanced CO2 capture performance and cost-effectiveness, the specific impurity-derived dopants governing cyclic stability and sorption kinetics still remain poorly understood. In this contribution, we reveal that Al doping (Si/Al = 3.76–5.68) improves cyclic stability by serving as both structural separator and support, while Na doping (Si/Na ≤20.6) enhances sorption capacity and kinetics. DFT simulations confirm Al reduces surface energy while Na increases exposure facet adsorption energy. Perlite is selected as the optimal silicon source from six minerals, and the derived perlite-Li4SiO4 demonstrates superior CO2 sorption capacities of 33.9 wt% at 650 °C and 8.3 wt% at 400 °C under 100 vol% CO2, maintaining 92% initial capacity even under low CO2 concentrations (20%). Remarkable regeneration stability is observed through 100 cycles with only 1.7 wt% capacity loss. Lower activation energy (chemisorption phase) and 1.73 GJ/t CO2 regeneration heat confirm its industrial viability. The impurity-doping synergism elucidates a self-reinforcing rationale for engineering stable high-temperature CO2 capture materials with operational durability. Ph.D. candidate Hu Xixuan from the College of Environmental Science and Technology serves as first author, with Professor Wang Qiang and Associate Professor Huang Liang as corresponding authors. Beijing Forestry University is the signature unit of the first author.
This research was funded by the National Key Research and Development Program of China (2022YFB4101702), the National Natural Science Foundation of China (52225003, 42075169 and 52106072), Engineering Research & Innovation Team Project of Beijing Forestry University (BLRC2023B04).
Paper link: https://doi.org/10.1016/j.cej.2025.165029
Written by Hu Xixuan, Huang Liang
Translated and edited by Song He
Reviewed by Yu Yangyang












